| Promote process management and pursue excellent quality; Continuous improvement to provide customers with satisfactory results。
|
 | Institutional and personnel management The company is equipped with management personnel at all levels who have certain professional knowledge, production experience, and organizational capabilities, as well as a certain proportion of technical personnel who are suitable for production management and quality management. The workshop production operators have a high school education or above, have undergone professional training and GMP knowledge training, and are certified to work after passing the assessment. |
 | Factory and facility management The company's factory buildings and facilities meet the requirements and have been verified. The areas are clearly distinguished, the production environment is clean, there are no sources of pollution around the factory area, and the water quality and noise meet the requirements of production hygiene. The factory buildings are arranged reasonably according to the process flow, with separate personnel and logistics. The work site is spacious, and various functional rooms are set reasonably. During operation, it is easy to use and can avoid confusion and cross pollution. |
 | Equipment management The production equipment and its supporting public equipment shall be reasonably arranged according to the process requirements, and the materials used shall be materials that do not undergo physical adsorption or chemical reactions with the production products. The surface shall be smooth and easy to clean. The instruments, meters, gauges, scales, and other equipment used for production and inspection meet the requirements of production and quality inspection in terms of applicability and accuracy. They are calibrated regularly and have clear qualified status marks, greatly improving production efficiency during operation. |
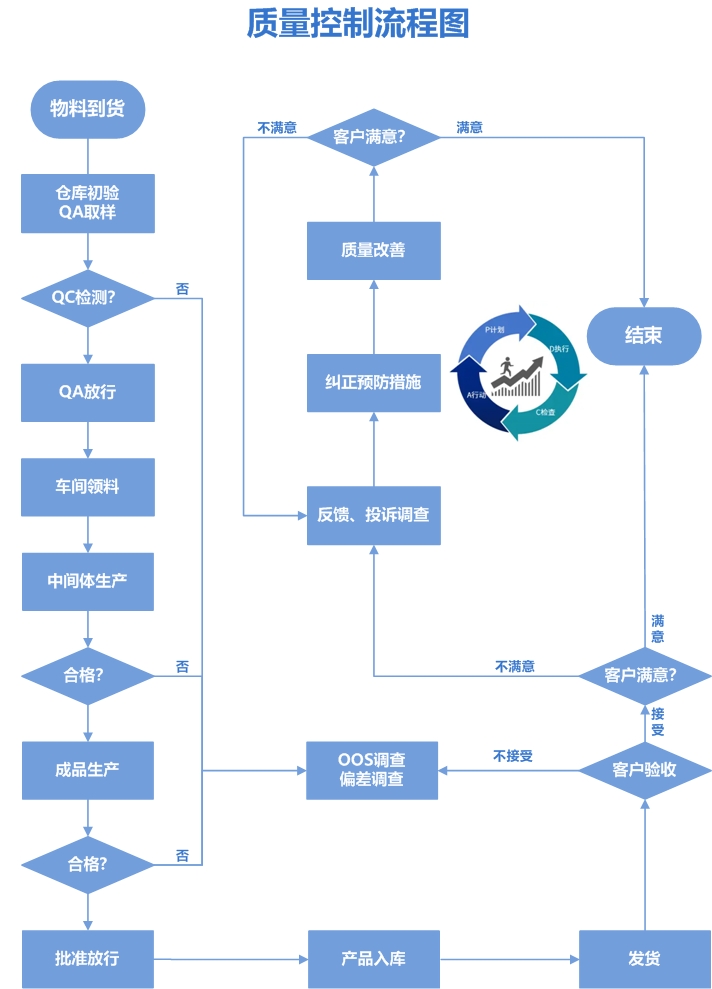
 | Material and product management The company is equipped with a complete and comprehensive document system and operating procedures, which can be correctly implemented and recorded, ensuring correct reception, storage, distribution, use, and shipment, and preventing pollution, cross contamination, confusion, and errors; The source of raw and auxiliary packaging materials used is stable, and quality evaluations are conducted for the determination and change of suppliers. The transportation of materials and products can ensure the quality of drugs; Each receipt of materials is recorded, covering the 8 requirements specified in Article 106 of GMP. |
 | File management The company has established a comprehensive quality management document system, which is suitable and in line with the actual production quality management. It can correctly guide actual production and strictly follow GMP requirements during the production process. All activities can be proven through documents and records, and there are procedures and records for the development, review, approval, distribution, retrieval, and destruction of documents. |
 | Production management Strictly organize production in accordance with approved quality standards and process procedures, weigh and review batches according to production instructions, and ensure that documents can correctly guide production work. Intermediate products are managed by dedicated personnel, and there are clear status markings on production sites, equipment, and containers. Products of different varieties or different specifications and batch numbers of the same variety can be thoroughly cleared at the end of production, with clearance records included in batch production records. |
 | Quality control and Quality assurance The quality department of the company consists of a quality assurance department and a quality control department, responsible for supervising the entire production process and quality control; Establishing QA in production workshops, warehouses, and other departments can effectively fulfill the quality supervision function throughout the entire production process. Management documents and SOPs can correctly guide actual work. Management personnel at all levels check the quality layer by layer. Unqualified raw materials are not put into use, unqualified intermediate products do not flow into the next process, and finished products that do not meet quality standards do not leave the factory; QC is currently equipped with multiple main inspection instruments including high-performance liquid chromatography, liquid chromatography, acidimeter, polarimeter, moisture analyzer, stability test box, etc., which are compatible with our company's existing production scale, variety, and inspection requirements. |